FAQ
Augma has 2 main products:
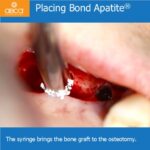
Bond Apatite® is a long term space maintainer, it can be used on any type of bony defect and comes in 1cc syringes. The composition is 2/3 Biphasic Calcium Sulfate and 1/3 of Hydroxyapatite particles which slow down the resorption process. After 3 months the result is ~90% patient own bone.
3D BOND+™ is a short term space maintainer, it can be used on socket cases with 4 walls and with a missing buccal plate, it comes in 0.5cc syringes. The composition is 100% Biphasic Calcium Sulfate. After 3 months the result is 100% patient own bone.
For graft protection use the Augma Shield™. When placed on top of the graft, it protects it and allows soft tissue to proliferate over the Augma graft, and for healing to take place.
There is no problem if the material breaks during suturing; simply place a dry gauze above, press firmly for one second, and continue to suture.
Can membranes be used with biphasic calcium sulfate?
With Augma bone graft cement, there is no need for membranes. The cement can set and harden in situ. As such, it acts as a graft and a barrier at the same time. The exceptional biocompatibility and bacteriostatic nature of calcium sulfate provide a synergic matrix for soft tissue to proliferate safely and rapidly. Hence, invasive surgery is not needed to gain a tension-free flap, nor for primary closure, which is not mandatory. Placing a membrane above the material will delay the healing and will force traditional invasive surgical protocols to be performed, and thus, also expose the patient to all known traditional complications.
Can the use of a membrane interfere with the initial neovascularization and neo-osteogenesis during the first post-surgical days?
The use of membranes inhibits soft tissue proliferation above the bone cement. It blocks the periosteum and impairs its osteoprogenerativity. In addition, if membranes are used, traditional bone augmentation rules such as tension-free flaps, and primary closure must be followed.
Are membranes recommended in edentulous ridge augmentation cases?
No membrane is required with Augma bond cement.
If primary closure in a case of missing buccal plate defect could not be obtained, can collagen membrane be used?
No, using a membrane is not recommend. Perform augmentation, close the flap, and suture. If the graft is exposed more than 3mm, secure a collagen sponge on top as shown in the protocols.
What types of sutures are recommended?
Sutures can be resorbable or nonresorbable. If resorbable sutures are used, avoid fast resorbable sutures that resorb in less than two weeks, such as the Chromic Gut. When using nonresorbable sutures, they should be taken out 10-14 days post-op.
Can Augma bone graft cement be mixed with FDBA?
There is no need to mix Bond Apatite® with other grafts since it is a pre-made composite graft. If one chooses to make their own made cocktail, 1cc 3D Bond can be mixed with any particles of other grafts according to the mixing protocol.
Can Augma bone graft cement be mixed with autogenous bone particles to improve the result?
There is no need, as Augma bone graft cement is a bioactive material that resorbs and facilitates the formation of a new bone.
Can PRF be used with Augma bone graft cement?
There is no need for PRF. Soft tissue healing is optimal due to the characteristics of the material and its high ability to promote angiogenesis.
Can osseodensification work with Augma?
Yes; make sure to respect osseodensification protocols and bone cement protocols.
Can Augma bone graft cement be used with immediate implant placement?
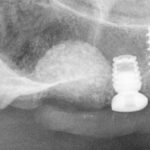
Yes, as long as the implant has a good primary stability. If the implant has exposed threads in the buccal aspect, place the implant 1-2 mm below the crest level. Cases with exposed threads that are within the bony envelope are more predictable than cases where the exposed threads are outside the bony envelope.
Can Augma bone graft cement be placed directly over exposed threads of implants to grow bone as the implant integrates?
Yes, the transformation of the material into native bone can assure decent osseointegration. In cases of exposed threads, make sure to position the implant 1-2 mm below the crest level. It is vital to be aware that the integration process is much more predictable when the exposed threads are within the bony envelope frame, due to the protection afforded by the adjacent bony walls. If any exposed threads are outside the bony envelope frame, movement of the soft tissue during the healing phase can lead to threads being exposed at the cervical area.
What about immediate loading during the placement of the implants?
In most cases, this is feasible if there is good primary stability. Judgment should be based on experience with similar conditions of primary stability and quality of the native bone when no augmentation was performed.
How long should one wait before uncovering implants?
In most cases, implants can be placed after 3-5 months. Loading the implants after the augmentation procedure depends on the size and morphology of the defect. For example, when there is no bony wall support, such as in lateral augmentation, the recommended time frame is 4-5 months to allow for improved maturation of the grafted site.
What is the quality of the bone after five years or more?
The material does not integrate with the bone. Instead, it resorbs and transforms completely and regenerates the patient’s own bone. After the bone formation, the natural biological bone remodeling process takes place continuously, while the bone continues to calcify and mature throughout the years like any living bone.
Is there a specific implant torque used in Augma-grafted bone?
There are no specific torque indications. Use the same torque as with native bone according to the bone type.
How thick should the graft material be applied on top of the implant at the initial placement?
Cover the socket and the implant and slightly overfill (1-2 mm).
Can there be an issue if waiting for more than 4-5 months between augmentation and implant placement? (For example, one year)
There is no upper limit on time between augmentation and implant placement. The bone will continue to maturate.
Can the material be used after the implant has been placed and integrated, in order to thicken the bone at the second surgery site?
Of course; this is an excellent and effective way to enlarge the bony width around the implants.
Can removable dentures be used while healing?
Any removable appliance is contraindicated since movement and pressure will jeopardize the outcome.
What about an interim denture? How can a patient go without teeth for months?
It is not easy to convince the patient to go for three months without teeth, but this is the best option to achieve bone growth. In some cases, if 2-3 temporary narrow implants can be placed in the anterior part and loaded immediately, this can provide the patient with a fixed temporary partial bridge in the anterior zone. Patients will accept this more readily than no teeth at all. If this option is not feasible, however, the patient should be supported in managing the entire healing period without teeth, as any removable denture will risk the outcome.
When can a patient start wearing temporary dentures after implant placement?
Temporary dentures can be used starting 2.5-3 months from the day of surgery
Is it recommended to decorticate the bone before placing Augma bone graft cement?
Decortication is recommended in cases where there is a cortical plate. The decortication holes should penetrate the entire cortex to reach the spongy bone.
Is it recommended to perforate the socket walls with a bur to stimulate bleeding?
In most cases, there is no need; there may be a need in very extreme cases, when the socket looks very dry.
What if perforation does not produce bleeding?
Perform deeper decortications and wait a bit longer until blood appears.
What is the recommended time to wait before reentry?
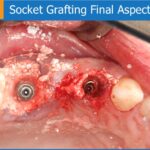
This depends on the case. In cases of socket grafting, three months are usually sufficient; in lateral augmentation, 4-5 months is recommended.
What type of bone density should one expect to see after four months?
In most cases, one can expect to see type 2-3 bone after four months.
Is Augma-grafted bone more vulnerable to developing peri-implantitis?
The material completely transforms into the patient’s bone. Therefore, it behaves like any vital natural bone and carries no increased risk of peri-implantitis.
What is the quality of the bone after five years? Does it become weaker?
No. With time, the bone becomes more mature due to calcification and the natural biological remodeling process.
How long does it take Augma bone graft cement to completely resorb?
This depends on the morphology of the site, blood supply, age, and other factors. In socket grafting, according to the histological evaluation taken after three months, 90% of the graft is replaced by bone. After eight months, 97% of the graft is completely replaced. In lateral augmentation, the process will take longer.
What are some common reasons for failure?
Failures may occur when bone cement protocols are not followed or respected. Trying to apply traditional grafting protocols with bone cement, such as using membranes and tension-free flaps, may lead to compromise of the outcome. Failure can also be seen in cases of heavy smokers, medically compromised patients, and patient non-compliance.
Due to the bacteriostatic nature of the material and the minimally-invasive surgery instructed by the protocols, it will be extremely rare to encounter similar complications to those encountered in traditional grafting, such as infection in case of exposure or loss of hard and soft tissue. However, in cases where the material is placed in an infected sinus or an acutely infected site, it can become contaminated.
In cases where the site is not pre-infected, the worst-case scenario is mostly not gaining the expected volume. In this case, re-augmentation is the best solution.
Some failures are related to the application of the material. For example, in cases where the cement is not well compacted in place, one might encounter soft tissue with dispersed granules instead of solid bone.
If the material is left unprotected and exposed for more than 3 mm, the material can wash out.
In a socket, if the material is not well compacted at the cervical area, the patient might experience discomfort after 3-4 days.
Cases of vertical augmentation or peri-implantitis cases are unpredictable and carry a higher risk of failure.
What is the histological difference between Augma bone formation and classic allograft formation?
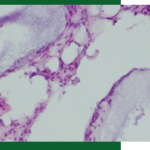
The histology slides of Augma bone graft cement do not present any integration of the graft since the graft resorbs and new bone is formed with osteocytes within the bone. This fact reflects that the new bone is vital. With allografts, there is an integration of the allograft particles with the surrounding bone. However, there are no osteocytes within the integrated allograft particles, which is a confirmation that those particles are sterile sequestrum and not vital bone.
Is there a risk of development of granulation tissue after using Augma bone graft cement?
If the graft is not well compacted and protected at the cervical zone of the socket according to the protocols, the end result may not lead to solid bone.
What is the reason for the discrepancy between the CBCT appearance and the bone density during reentry?
A CBCT image with traditional bone grafting appears radiopaque at all times from day of placement along the course of healing, as well as during reentry, due to the fact that the graft integrates with the bone and is not replaced by it. Therefore, the radiopacity seen in CBCT reflects more the presence of the graft in the site and less the newly-formed bone in between the graft particles. In contrast, when biphasic calcium sulfate bone cement is used, complete resorption of the graft takes place, and the augmented site transforms into a new vital bone. Therefore, in 3-4 months, it is a young bone that might look less radiopaque than the surrounding native bone.
There are ‘white’ particles three months post-op; are these HA (hydroxyapatite)?
Yes, these particles are the large HA particles that comprise the remaining 10% of the composition of the Bond Apatite® composite graft cement. At this stage, they are in the process of resorption.
What are the instructions for the patient immediately post-op?
On the day of the surgery, avoid mouth rinse. In the first 2-3 weeks, use a relatively soft diet and try to avoid unnecessary contact with the tongue or finger.
For 10-14 days after the surgery, perform mouth rinse two times a day with chlorhexidine gluconate 1.2-2% or saltwater. Do not gargle; keep the solution in the mouth for 1-2 minutes and then spit it out. Follow post-surgery medication and follow-up visits, according to the medical requirements.
What is recommended in an upper molar extraction site without primary closure?
This type of site must be protected with a secured collagen sponge/plug or Augma Shield, according to the protocols.
For how long is a soft diet recommended?
A soft diet is recommended for the first 2-3 weeks post-op.
Are tenting screws for graft stabilization recommended if the defect is large?
No, tenting screws should not be used.
Can Augma bone graft cement be placed over the top of a perforation, or does the perforation need to be closed first?
When performing socket preservation, after the extraction and preparation for a future implant, a small perforation may be visible on the buccal or on the base of the socket. Augma can be placed over the perforation simultaneously with the closing of the perforation.
Can Augma be used immediately after extractions when a patient has periapical infections?
In cases of acute infection, no augmentation should be performed until all symptoms regress. In symptomless chronic situations, augmentation can be performed after proper debridement.
Is there a time frame in which Augma graft cement can be used in acute infection?
No augmentation should be performed until all signs and acute symptoms have disappeared. This may take about 2-3 weeks, and sometimes even longer, after antibiotic administration and elimination of the cause. Therefore, there is no definite, exact time frame. The decision should be made at least two weeks after the recession of the acute symptoms.
Can Augma be used for peri-implantitis treatment?
There is no predictable protocol at the moment for peri-implantitis treatment using Augma. This includes cases with less severe mobility.
Can Augma bone graft cement can be used for grafting of periodontic defects around teeth or implants?
Yes, the flap reflection, graft placement, and soft tissue closure are the same as the protocols for socket grafting with flap reflection.
How long has the material has been on the market?
Calcium sulfate has been in use for more than 120 years—the patented biphasic calcium sulfate, since 2010.
Can the ‘tunnel technique’ work with Augma bone graft cement?
Yes; however, make sure to compact the cement properly in place.
What type of flap is needed, partial-or full-thickness?
A full-thickness flap is necessary.
Is a full periosteum release recommended to allow tension-free closure, or how much should one release?
Do not perform any periosteum-releasing incisions. The protocols for Augma bone graft cement instruct to maintain tension on the flap during closure. In this way, the flap is minimally reflected and stretched for closure.
Why should sutures not be engaged with the muscles?
The flap is the part that should not be connected to the muscles; therefore, the flap is minimally reflected, as explained in the protocols. The sutures are not the issue.
If the vertical incision is more than 3mm past the mucogingival line, is it possible to suture without engaging the muscles?
The sutures do not have any influence on muscle engagement. The flap design and the way it is reflected, together with a lack of releasing incisions, are the factors that prevent muscle engagement.
Can tension-free flap closure be used, or is stretching the flap with tension necessary?
With Augma bone graft cement, no horizontal releasing incision is recommended to gain a tension-free flap. The flap should be under tension during the closure. Therefore, the flap should be minimally reflected according to the protocols while the flap closure is accomplished by stretching.
Should the flap be stretched if the MGJ is closer to the ridge and reflection is beyond 3 millimeters?
Yes, the flap should be stretched in this case.
Can soft tissue grafting be performed before bone grafting if the tissue phenotype is thin?
Yes, soft tissue grafting can be performed in this case.
Is the ‘brushing technique’ recommended to release the soft tissue without vertical incisions?
No, this technique is not recommended.