Calcium sulfate
More than 100 years of documented clinical success in bone augmentation
Biphasic Calcium Sulfate is composed of two phases of the well known Calcium Sulfate. Calcium sulfate (CS) features a unique position among all regenerative bone graft substitutes. With more than 100 years of documented clinical success, (Dreesmann 1892, Thomas and Puleo 2008) it has a longer history of clinical use when compared to most currently available biomaterials.
CS is widely recognized as a well-tolerated bone regeneration material. It undergoes virtually complete resorption in vivo and has consistently been considered as highly biocompatible, osteoconductive, and easy to use (Boden, 1999 or more recently by Pietrzak and Ronk, 2000; Ricci et al., 2000; Tay et al., 1999; Thomas et al., 2005; Thomas and Puleo, 2008).
The raw material, medical grade calcium sulfate is clinically used in 2 different forms differing in their individual (crystal) water content and their physicochemical behavior. (Anusavice 2003).
3D Bond™ and Bond Apatite® Optimized Application Profile for usage in dental and maxillofacial surgery
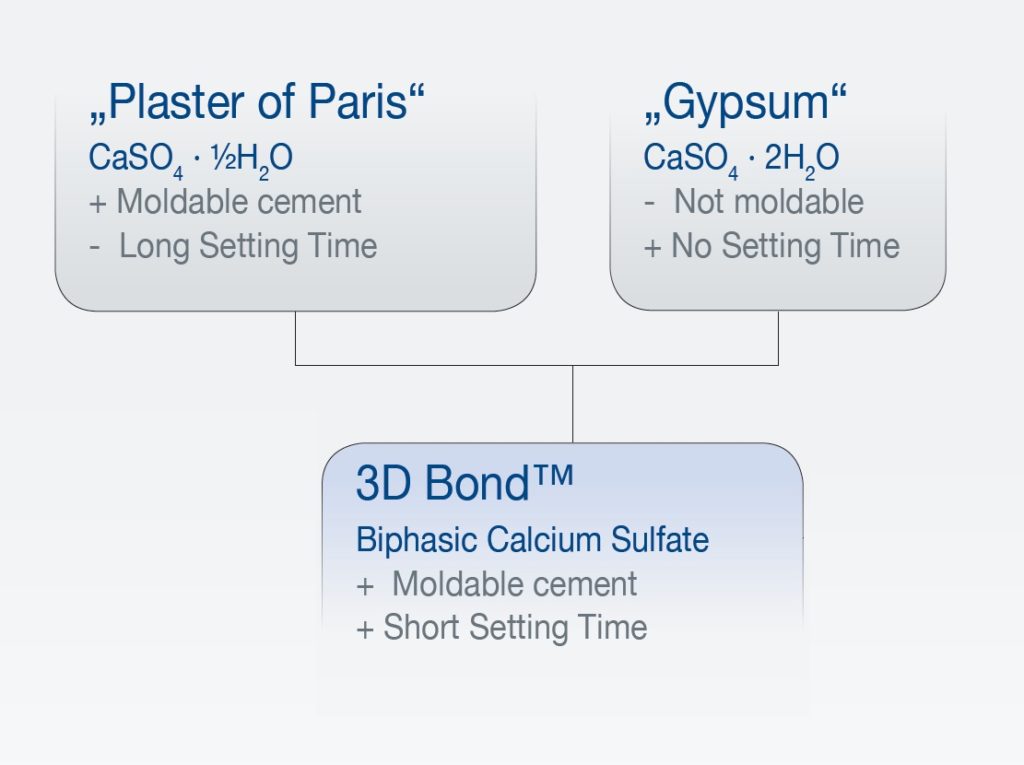
3D Bond™ and Bond Apatite® represent the next generation of calcium sulfate-derived bone graft materials. They are self-reinforced graft cement based on the concept of using highly pure biphasic Calcium Sulfate (BCS). Using both phases of calcium sulfate (hemihydrate and dehydrate) in a defined granular formulation provides significant advantages, such as improved handling from the materials’ moldability as well as accelerated setting capabilities, even if blood and saliva are present. This also makes for easy and safe use in many typical dental indications.
3D Bond™ and Bond Apatite® feature the following characteristics as prerequisites for your clinical success:
- Better Application: Moldable cement for facilitated and precise augmentation.
- Faster Regeneration: Optimal graft characteristics for fast and complete vital bone formation.
- Proven & Safe: Well-documented synthetic technology for optimal graft safety and biocompatibility.
3D Bond™ is a graft binder cement made of pure biphasic calcium sulfate (BCS). It is delivered in a syringe containing the granulated powder. After mixing with liquid, 3D Bond™ can be directly injected into the graft site, either as a stand-alone graft or in a mixture with any bone substitute material.
Bond Apatite® is a bone graft cement combining 3D Bond™ with Hydroxyapatite granules.
The BCS matrix feature complete resorption between 4-10 weeks. The slow-resorbing hydroxyapatite granule matrix serves as longer range space maintainer for optimized volume control.
Bond Apatite® is delivered in a specially designed ready-to-use “All in One” syringe containing the granulated powder and physiological saline. Mixing the powder component with the liquid in the driver results in a viscous composite that is suitable for direct injection into the graft site.
The cement property of Bond Apatite® enables the entire graft placement and stabilization to be completed in less than one minute by the 3 short consecutive steps Place-Press-Close.
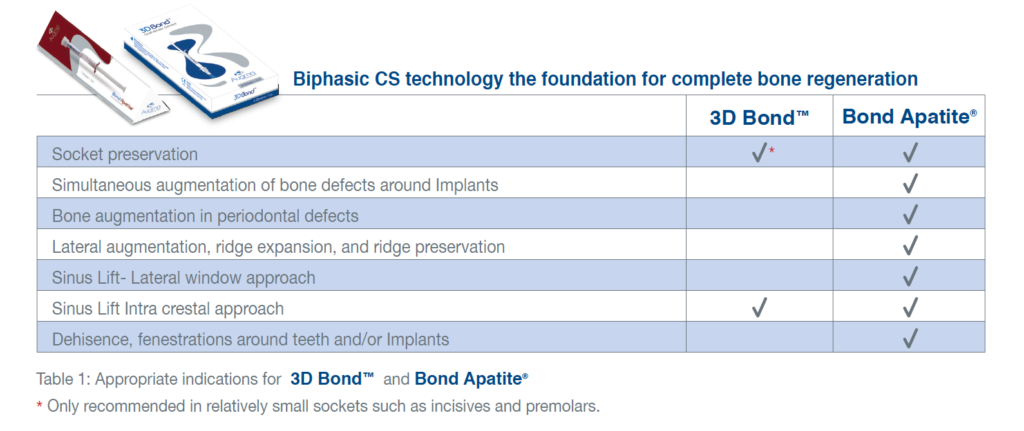
Indications
Biphasic CS technology as a base for complete bone regeneration
3D Bond™ and Bond Apatite® are indicated for use in following indications:
100% Primary Volume Stability
Moldable cement for ideal initial space maintaining function
3D Bond™ and Bond Apatite® are self-reinforced graft binder cement and bone graft cement, respectively, based on the concept of using highly pure biphasic Calcium Sulfate (BCS).
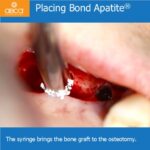
After mixing the BCS derivate with sterile saline, an injectable putty/moldable cement is formed and 3 consecutive steps should be followed: Place -Press -Close. The putty can be easily applied and bond to the bone defect. Due to the cement-like consistency, the defect configuration can be accomplished very precisely.
After a short settling period, the cement hardens in situ to provide a 100% mechanically stable environment for facilitated membrane application (if required) and wound closure.
Due to the material composition, Bond Apatite® is designed as a stand-alone-bone graft material. The combination with an appropriate barrier membrane is optional and might be indicated, especially if high graft resorption is expected.
3D Bond™ can be mixed with any existing bone graft substitute to profit from both the BCS graft binder cement properties as well as from a certain resorption pattern of the individual bone graft substitute material.
The entire graft placement and stabilization is carried out by 3 short consecutive steps: Place-Press-Close
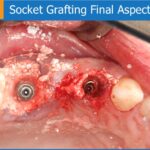
Fig. 1: “Place”-precise application of the cement to the defect resulting in a stable graft positioning.
Fig. 2: ”Press”- after graft placement, pressing with a dry gauze generate a fast setting also in the presence of blood and saliva. After setting: perfect and stable graft positioning as a prerequisite for recontouring the alveolar crest.
Fig. 3: ”Close”- soft tissue closure can be done directly above the harden graft.
* (membrane coverage before soft tissue closure is not necessary).
Faster Regeneration
Optimal graft characteristics for fast and complete vital bone formation
3D Bond™ and Bond Apatite® feature both micro- (0-10μm) and macroporous (50-500μm) structure paired with a high overall porosity of more than 40%. (Fig. 4)
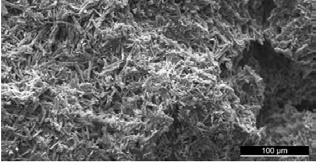 Fig. 4: SEM of 3D Bond™ post-setting structure, composed of needle-like crystals with a high micro- and macroporosity.
Fig. 4: SEM of 3D Bond™ post-setting structure, composed of needle-like crystals with a high micro- and macroporosity.
These material characteristics are prerequisite for BCS’s excellent scaffolding characteristics needed for new bone formation. The micro-pores allow growth factors infiltration, and macro-pores allow osteoblasts infiltration and angiogenesis.
By that, BCS is differentiating significantly from many other synthetic graft materials that usually only barely contain a microporous system.
After implantation, CS binds to the adjacent bone (Coetzee 1980) and features a fast dissolution. It leaves behind calcium phosphate deposits that stimulate new bone growth accompanied by increased neoangiogenesis, both prerequisites for a 100% vital and well-vascularized bone structure. (Ricci 2000, Strocchi 2002, Turri & Dahlin 2014). The rate of new bone formation for CS was shown to be much faster than for many kinds of typical bone graft substitute materials, regardless of their origin. (Turri & Dahlin 2014, Toloue 2012, Collins 2014).
In an animal trial (rabbit maxilla) Dahlin and Co-Workers histologically compared the dynamics of bone healing response between 3D Bond™ and deproteinized bovine bone mineral (DBBM) particles in a contained guided bone regeneration setting utilizing a membrane as a barrier.
% New Bone Formation
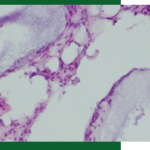
Figure 5: Histological Examination of new bone formation at different healing periods.
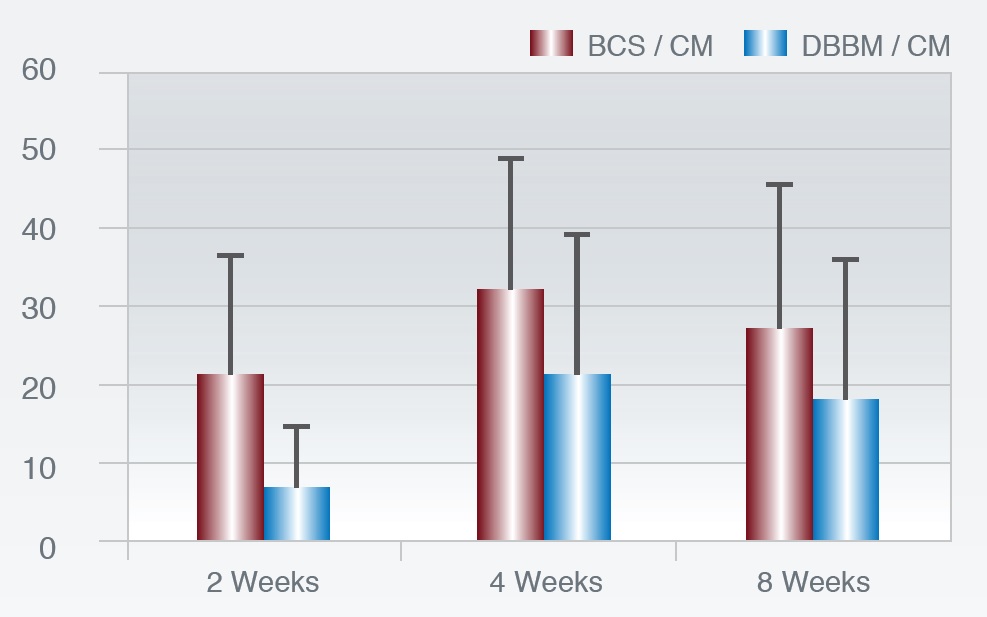 Fig. 5: The 3D Bond™ group showed significantly more bone regeneration at all three healing periods vs. The DBBM group. Total resorption of the BCS material was seen already at 2 weeks, while only minor resorption could be seen of the DBBM particles.
Fig. 5: The 3D Bond™ group showed significantly more bone regeneration at all three healing periods vs. The DBBM group. Total resorption of the BCS material was seen already at 2 weeks, while only minor resorption could be seen of the DBBM particles.
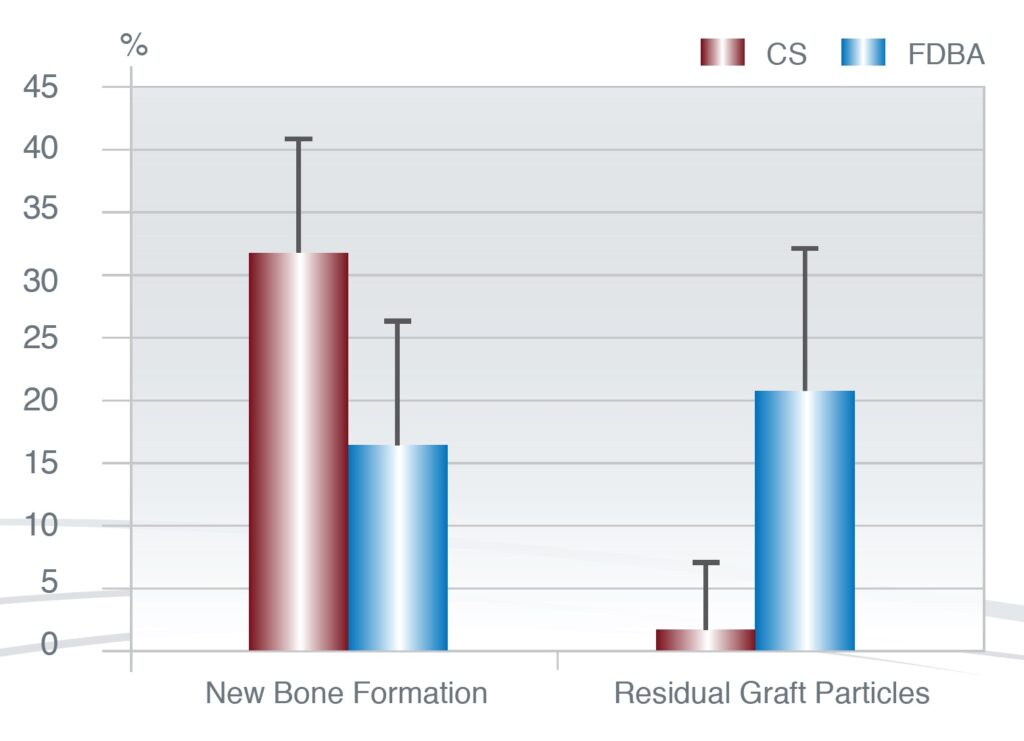
In a comparative clinical trial, Toloue determined whether calcium sulfate (CS) is as effective as freeze-dried bone allograft (FDBA) in preserving postextraction ridge dimensions and evaluated the amount of new bone formation and graft clearance through histologic analysis. (Toloue 2012)
Both materials showed similar ridge preservation capabilities 3 months after bone augmentation. Extraction sockets grafted with CS resulted in the significantly more new bone formation and significantly less remaining bone graft particles.
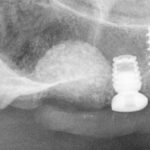
Human Histology 3 months post-op
Figure 6: Histological Examination of new bone formation and remaining bone substitute 3 months after socket augmentation.
Proven & Safe
Well-documented synthetic technology for optimal graft safety and biocompatibility.
Based on a well-documented fully synthetic technology concept – Optimal graft safety and biocompatibility.
The BCS products are based on highly pure medical grade calcium sulfate and represent a synthetic grafting concept without any risk of disease transmission.
After implantation, calcium sulfate is completely dissolved into its component elements naturally found in the body; this makes this bone graft material well tolerated and non-immunogenic. CS is used as a bone substitute material in all medical disciplines for >100 years (Dreesman 1892), and no adverse reactions or failures to heal have been reported in the literature so far. (Thomas and Puleo 2008)
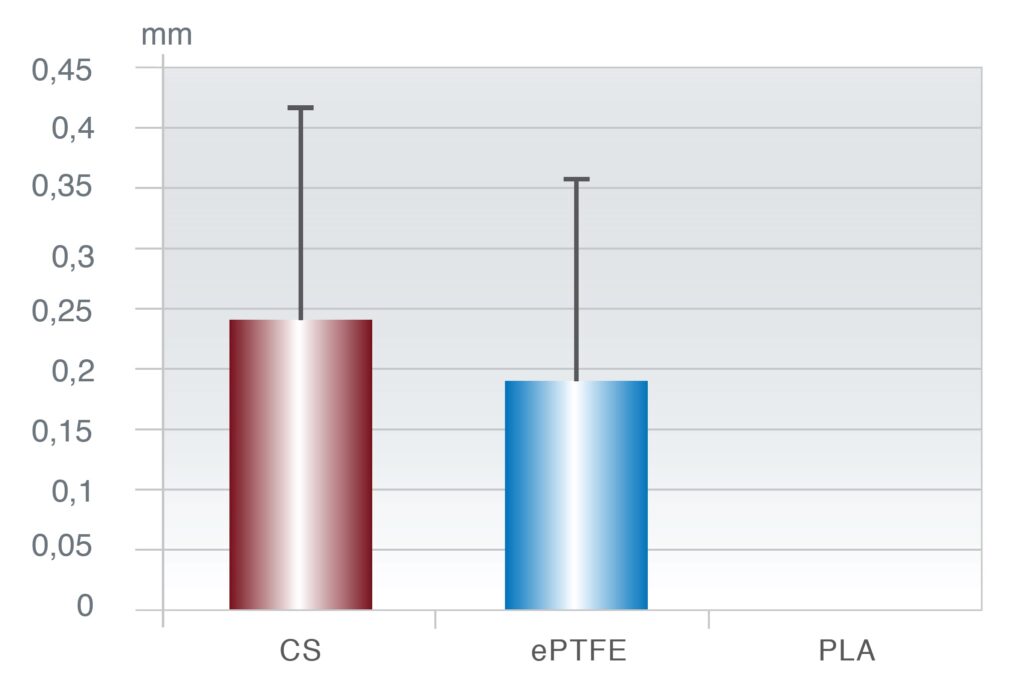
In an in vitro study, Payne et al. assessed the ability of cultured human gingival fibroblasts to migrate over various barrier materials in response to a chemotactic stimulus. (Payne) The materials tested were ePTFE, poly(lactic acid) (PLLA), and CS. The cells were able to migrate further on CS than on the other materials tested. Scanning electron microscopic examination of the cells indicated that cellular morphology appeared normal on the CS substrate, whereas cells on ePTFE and polylactic acid barriers exhibited abnormal morphology and did not appear to be migrating.
Fibroblast Migration Distance (mm)
Figure 7: Fibroblast Migration on different synthetic biomaterials.
Technology
Biphasic Calcium Sulfate as 2nd generation technological breakthrough in the long history of CS bone regeneration
The first documented use of CS as augmentation material dates back to 1892 reported by Dreesmann to obliterate bone cavities caused by tuberculosis (reviewed by Peltier, 1961; Peltier and Speer, 1981).
Since then, researchers and clinicians have continued to explore the effectiveness of the material in various applications. The long clinical history as an augmentation material was reported and published in thousands of scientific articles during 120 years of research, not only in dental and maxillofacial applications (Coetzee, 1980), furthermore in orthopedic, plastic surgery, oncology, revision arthroplasty and spinal arthrodeses (Bucholz, 2002).
The raw material, medical grade calcium sulfate is clinically used in 2 different forms differing in their individual (crystal) water content: (Anusavice 2003)
a) Calcium sulfate hemihydrate (CaSO4 · ½ H2O), also known as „Plaster of Paris“
b) Calcium sulfate dihydrate (CaSO4 · 2 H2O), also known as Gypsum“
When the CS-hemihydrate is mixed with water, it forms a moldable paste (cement) that sets after a certain time interval. By that, the other CS form, the respective dihydrate is formed: CaSO4 · ½H2O + 1½H2O –> CaSO4 · 2H2O
The complete setting process of CS Hemihydrate needs approx. 20 min and is controlled by the formation and growth of CS dihydrate crystals. In an in vivo scenario, proteins and other biological macromolecules may further retard the setting time to up to 200 min. This is impeding the application procedure as well as the clinical performance of a typical dental application significantly. (Ricci 2000)
Although Orsini and co-workers have shown that there is no difference in the bone healing pattern between preset CS dihydrate granules and moldable CS-hemihydrate cement. (Orsini 2004), by using preset CS dihydrate the surgeon loses advantageous handling properties of the moldable cement derivative.
By making use of the biphasic calcium sulfate concept in 3D Bond™ the previous setting issues can be solved: The CS hemihydrate component is controlling the cement consistency and moldability characteristics. The dihydrate component is regulating the setting properties. Using this formulation, the setting process can be reduced from about 20 minutes to 3 minutes, also under in vivo conditions in presence of blood and saliva.
The key performance criteria of CS-derived graft materials that were shown in numerous studies is the fast and effective vital bone formation as a result of the rapid dissolution in vivo. (Thomas and Puleo 2008, Toloue, Dahlin) These characteristics provoke advantageous clinical results in small and well-contained bony defects like extraction socket filling. (Toloue, Guarnieri)
Figure 9: Unique structure of bi-phasic calcium sulfate seed particles, composed of both hemihydrate and dihydrate phases of calcium sulfate.
Nevertheless, when the material shall be applied in larger bone defects with less graft containment, the fast dissolution of CS may lead to a lack of stable osteoconductive scaffold after a certain time, thus losing its mechanical properties. (Walsh) By that, the preservation of the augmented graft volume may be compromised over time.
In order to improve the scaffolding properties of calcium sulfate, it has been successfully used with many kinds of other bone grafts. (Stubbs 2004, Urban 2007)
Figure 10: Bond Apatite featuring the concept of bone graft cement.
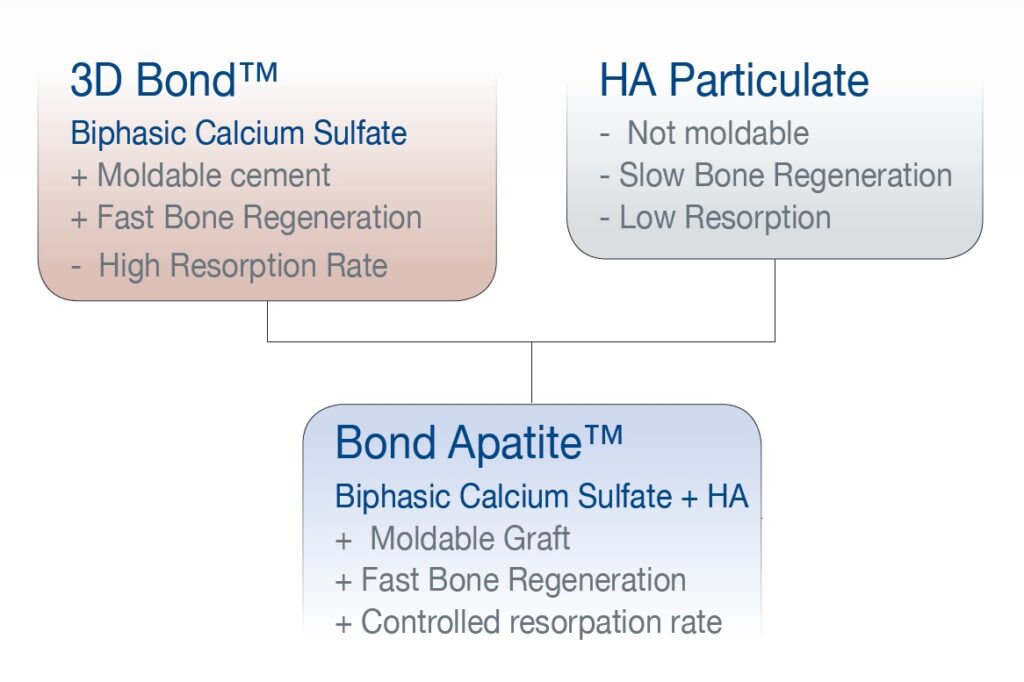 Fig. 10
Fig. 10
Bond Apatite® is composed of biphasic calcium sulfate and synthetic hydroxyapatite granules. With this formulation, the concept of graft cement can be successfully applied for a wider range of indications.
The BCS affects an injectable graft cement and features fast resorption and new bone formation. The hydroxyapatite granules provide a slow-resorbing osteoconductive scaffold to preserve the augmented volume also over a longer time period.
References
- Dreesmann, H. über Knochenplombierung. Klinische Chirurgie, 1892;9:804-810.
- a) Thomas, M.V., Puleo, D.A., Calcium sulfate: A review. J. Long Term Eff. Med. Implants, 2005;15(69):599-607. b) Thomas, M.V., Puleo, D.A., Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. B Appl. Biomater., 2008 Nov 24. [Epub ahead of print]
- Peltier, L.F., The use of plaster of Paris to fill defects in bone. Clin. Orthop. Res., 1961;21:1-29.
- Peltier, L.F., Speer, D.P., Calcium Sulfate. In: Habal M. B., Reddi A.H. (eds) Bone grafts and bone substitutes, Saunders Co., Philadelphia, 1981:243-246.
- Coetzee, A.S., Regeneration of bone in the presence of calcium sulfate. Arch. Otolaryngol., 1980;106:405-409.
- Bucholz, RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;395:44-52.
- Boden SD, Stevenson S. Bone grafting and bone graft substitutes. Philadelphia: Saunders, 1999.
- Pietrzak, W.S., Ronk, R., Calcium sulfate bone void filler: A review and a look ahead. J. Craniofac. Surg., 2000;11(4):327-333.
- a) Ricci JL, Alexander H, Nadkarni P, Hawkins M, Turner J, Rosenblum SF, Brezenoff L, De Leonardis D, Pecora G. Biological mechanisms of calcium- sulfate replacement by bone. In: Davies JE, editor. Bone Engineering. Toronto: Em Squared Inc., 2000:332-344. b)Ricci, J.L., Report on the use of calcium sulfate cement with dental cement. In: Use of Calcium Sulfate Cement for Dental and maxillofacial Bone Repair, edited by Pecora, G. and De Leonardis, D., Elite Publishing, Rome, 7 pages, 2001.
- Tay, B.K.B., Patel V.V., Bradford, D.S., Calcium sulfate- and calcium phosphate-based bone substitutes: Mimicry of the mineral phase of bone. Orthop. Clin. North Am., 1999;30(4):615-623.
- Anusavice KJ. Gypsum products. In: Anusavice KJ, editor. Phillips‘ Science of Dental Materials. St. Louis, MO: Saunders, 2003:255-281.
- Strocchi, R., Orsini, G., Iezzi, G., Scarano, A., Rubini, C., Pecora, G. & Piatelli, A. Bone regeneration with calcium sulfate: evidence for increased angiogenesis in rabbits. The Journal of Oral Implantology, 2002;28:273–278.
- Turri A, Dahlin C. Comparative maxillary bone-defect healing by calcium-sulfate or deproteinized bovine bone particles and extracellular matrix membranes in a guided bone regeneration setting: an experimental study in rabbits. Clin. Oral Impl. Res. 00, 2014, 1–6 doi: 10.1111/clr.12425
- Toloue SM, Chesnoiu-Matei I, Blanchard SB. A clinical and histomorphometric study of calcium sulfate compared with freeze-dried bone allograft for alveolar ridge preservation. J Periodontol, 2012;83:847–855.
- Collins JR, Jiménez E, Martínez C, Polanco RT, Hirata R, Mousa R, Coelho PG, Bonfante EA, Tovar N. Clinical and Histological Evaluation of Socket Grafting Using Different Types of Bone Substitute in Adult Patients. Implant Dent, 2014;23:489–495.
- Payne JM, Cobb CM, Rapley JW, Killoy WJ, Spencer P. Migration of human gingival fibroblasts over guided tissue regeneration barrier materials. J Periodontol, 1996;67:236-244.
- Orsini G,Ricci J, Scarano A, Pecora G, Petrone G, Lezzi G, Piattelli A. Bone-defect healing with calcium-sulfate particles and cement: An experimental study in rabbit. J Biomed Mater Res B Appl Biomater, 2004;68:199-208.
- a) Guarnieri R, Grassi R, Ripari M, Pecora G. Maxillary sinus augmentation using granular calcium sulfate (surgiplaster sinus): Radiographic and histologic study at 2 years. Int J Periodontics Restorative Dent, 2006;26:79-85. b) Guarnieri R, Bovi M. Maxillary sinus augmentation using prehardened calcium sulfate: A case report. Int J Periodontics Restorative Dent, 2002;22:503-508.
- Walsh WR, Morberg P, Yu Y, Yang JL, Haggard W, Sheath PC, Svehla M, Bruce WJ. The response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin Orthop., 2003:228-236.
- Stubbs D, Deakin M, Chapman-Sheath P, Bruce W, Debes J, Gillies RM, Walsh WR, In vivo evaluation of resorbable bone graft substitutes in a rabbit tibial defect model, Biomaterials, 2004;25(20):5037-44.
- Urban, R.M., Turner, T.M., Hall, D.J., Inoue, N., Gitelis, S., Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin. Orthop. Relat. Res., 2007;459:110-117.